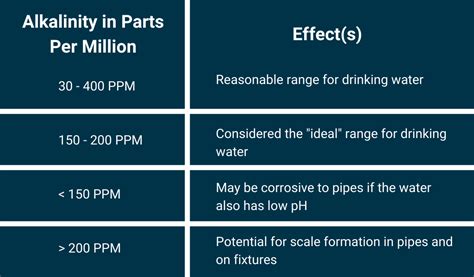
testing alkaline levels in the bottled water|alkalinity water chart : Brand Since pH can be affected by chemicals in the water, pH is an important indicator of water that is changing chemically. pH is reported in "logarithmic units". Each number represents a 10-fold change in the acidity/basicness of the water. Water with a pH of five is ten times more acidic than water having a pH of six. Importance of pH 6 de nov. de 2023 · Conta FF Gemada Grátis. 6 de novembro de 2023. Descubra a seleção exclusiva de contas FF gemadas gratuitas, oferecidas por veteranos do Free Fire. Essas contas estão abandonadas e algumas contém alguns itens que os jogadores esqueceram inúmeros triunfos e recompensas. É a sua chance de elevar sua experiência no Free .
{plog:ftitle_list}
Resultado da See Photos. Isadora Leite. View the profiles of people named Isadora Leite. Join Facebook to connect with Isadora Leite and others you may know. .
the perfect water chart
crystal hardness tester
Discover the bottled water pH levels of 33 popular brands in our comprehensive guide. Learn about acidity, alkalinity, and the potential health implications of your go-to bottled water. Stay informed and make the best .Water may have a low alkalinity rating but a relatively high pH or vice versa, so alkalinity alone is not of major importance as a measure of water quality. Alkalinity is not considered detrimental .Testing the pH of 20 brands of bottled water. Bottled Water has Low-Energy Risks for Our Bodies. Our bodies are made of energy at the source of all cells. Low-energy water (stagnant, "dead-water") makes the blood sticky and . There are many reasons why companies and scientists test pH levels in water. For drinking water, the pH range must be between 6.5-8.5. If the water is too acidic, it will likely taste and smell bad. It can also cause .
Since pH can be affected by chemicals in the water, pH is an important indicator of water that is changing chemically. pH is reported in "logarithmic units". Each number represents a 10-fold change in the acidity/basicness of the water. Water with a pH of five is ten times more acidic than water having a pH of six. Importance of pH
There is no legally enforceable standard for drinking water pH levels because pH is considered an aesthetic water quality. However, the U.S. Environmental Protection Agency (EPA) recommends a pH between 6.5 and 8.5 for drinking water. Since metals dissolve readily in acidic water, dissolved metals may be present in drinking water with a low pH . A pH of 7 is considered neutral, while anything below 7 is acidic, and anything above 7 is alkaline. Drinking water with a pH outside of the ideal range can lead to health issues and affect the taste of the water. Keep reading to learn more about the importance of pH and the best bottled water options for your health and sustainability. Key .The pH scale is a fundamental concept in water chemistry that is used to classify substances as being acidic, neutral, or basic. In this paper, we will delve into the pH scale and how it is used to classify substances based on their acidity or basicity. The pH scale is a logarithmic scale that ranges from 0 to 14, with 7 being neutral.Temperature will also affect the equilibria and the pH. In pure water, a decrease in pH of about 0.45 occurs as the temperature is raised by 25 °C. In water with a buffering capacity imparted by bicarbonate, carbonate and hydroxyl ions, this temperature effect is modified (APHA, 1989). The pH of most drinking-water lies within the range 6.5–8.5.
A professional water test provides the most comprehensive look at pH levels and what they mean for overall water quality. Read on to learn more about pH testing for your home’s water supply. Quick Facts About pH Water Testing. If you’re interested in the basics of pH testing, you’ve come to the right place. Here’s what to know:
Buy Alkaline Water (ph Test Kit) for Drinking Water Measures pH Level of Water More Accurately Than Test Strips pH Starter Kit Drops Easy to Use: Water Test Kits - Amazon.com FREE DELIVERY possible on eligible purchases I Tested The PH of EVERY BOTTLED WATER! Trying all the water brands and doing a a ph water test with Dasani, Evian, Voss, Aquafina and more! Leave a Like if .Conversely, low-pH water shows corrosive properties that can corrode metals and dissolve various substances, giving adverse consequences such as leaching metals. A reasonably stable pH level is needed to keep the water pH level neutral and usable. Water's pH level is usually affected by pollution, especially atmospheric carbon dioxide (CO2) gas.
safe alkalinity in drinking water
Collect a sample. Collect a sample of your water in a clean container. Make sure you get enough water to cover the whole strip, if that’s required by the brand you’ve chosen.; Dip the test strip. Dip the strip – partially or entirely, depending on brand – into the sample. While it may take a few minutes for the results to register, the actual dipping should only take a couple . The pH level tells you how acidic or basic your water is. The pH level of the water can affect your water pipes and how your water looks and tastes. If the pH of your water is too low or too high, it could damage your pipes. Heavy metals like lead can leak out of damaged pipes into the water and eventually make you sick. Test for total . PH Tests Show Most Bottled Water Testing PH to be Too Acidic This video shows testing of 20 brands of bottled water testing pH and theAlkaline Plus PH Pitcher by WellBlue. For this series I focused on testing the pH-levels of the most popular bottled water brands I could find and then theAlkaline Plus Pitcher because it is so highly effective, . You can also determine the cleanest drinking water by pH levels. Healthline discerns that a "safe" pH range is 6.5 to 8.5 — anything less could mean heavy-metal pollution.
Many people believe that drinking water that is specifically formulated to be alkaline, with a higher pH than regular tap water, supports the body in maintaining pH levels. They say it has the effect of easing the amount .
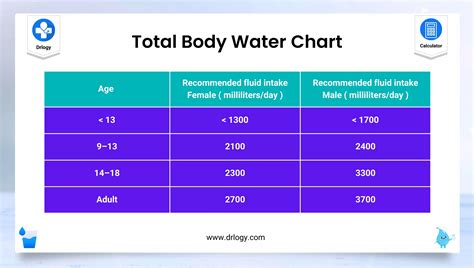
Drinking Water Quality: Testing and Interpreting Your Results (WQ1341, Revised Feb. 2022) File. Publication File: WQ1341. . A Quick Look at Safe Levels in Drinking Water (most are based on EPA recommendations) mg/l = milligrams per liter . The pH of water is a measure of acidity or alkalinity. The pH is a logarithmic scale based on a . Well Water: pH Level Range: Can vary significantly, usually between 6 to 8.5; Taste: Often described as clean and fresh; Spring Water: pH Level Range: Generally around 7.4; Taste: Often considered crisp and refreshing; Bottled Water: pH Level Range: Varies between brands, usually around 6 to 8.5; Taste: Depends on the specific brand and source
All living species require water to survive. The pH level of completely pure water is 7, which is exactly in the center of the scale, making it a neutral drink.However, most water includes particles that can raise the pH from 6.5 (acidic) to 8.5 (basic or alkaline).. Surface water systems typically have a pH range of 6.5 to 8.5, whereas groundwater systems have a pH range of 6 to 8.5. 🤔 What is pH? pH is a measurement used to classify how acidic or alkaline water is.The pH range is between 0 and 14, with 7 being “neutral” or “normal”. Water with a pH of lower than 7 is considered acidic, while water with a pH level that’s higher than the neutral pH of 7 is alkaline.The lower the water’s pH, the more acidic it is; the higher its pH, the more alkaline it is. The higher the score, the more alkaline a thing is. The lower, the more acidic it is. Alkaline water has a higher pH level than other types of drinking water, of which the municipally-deemed “safe” levels tend to clock in between 6.5-8. When consumed, alkaline food and beverages help to neutralize acidity in the body. As you can tell by the name, a pool pH testing kit is intended to be used to test the pH level of pool water. Pool water contains chemicals such as chlorine, sodium bisulphate, and sodium carbonate, and its value usually ranges from 7.0 to 7.6. . But aside from bottled alkaline water, you can also invest in an alkaline water machine or an .
Drinking water is best when it sits at a 7.0 pH. If the water’s pH goes below 6.5 or above 8.5, it likely contains high levels of chemicals or toxic metals. Several environmental factors invariably affect the water’s pH level. When water moves through the environment, it interacts with soil and bedrock. In its purest form, water has a pH of 7, which is at the exact center of the pH scale. Particles in the water can change the pH of the water, and most water for use has a pH of somewhere between 6 .
Alkaline Water (ph Test Kit) for Drinking Water Measures pH Level of Water More Accurately Than Test Strips pH Starter Kit Drops Easy to Use. 4.4 out of 5 stars. . Alkaline, Tap, and Drinking Water pH Level Test kit (WHT/100-125 Tests) 0.50 Fl Oz, More Accurate Than Test Strips, Made in USA, by A2O Water. 4.6 out of 5 stars. 1,415. .98 $ 6. 98.When we test drinking water samples we are looking for certain parameters. The levels of these parameters tell us if the water is acceptable and safe. . Some sources have naturally low pH levels due to soil, rock, and geology type in the catchment area of the source. In some cases, the pH of the water can dip below the lower regulatory limit . Learn more about water pH levels. 2. Why might someone want to test the pH of bottled water? Bottled water isn't always at a neutral pH. Some brands market alkaline water, while others may have acidic levels. Testing the pH of bottled water ensures you're aware of its quality and if it aligns with your health or taste preferences.
Resultado da 777 Online Casino. 777 is a part of 888 Holdings plc's renowned Casino group, a global leader in online casino games and one of the largest online gaming venues in the world. 888 has been listed on the London stock Exchange since September 2005. Everything we do is designed to give the .
testing alkaline levels in the bottled water|alkalinity water chart